
Electronic Data Collection
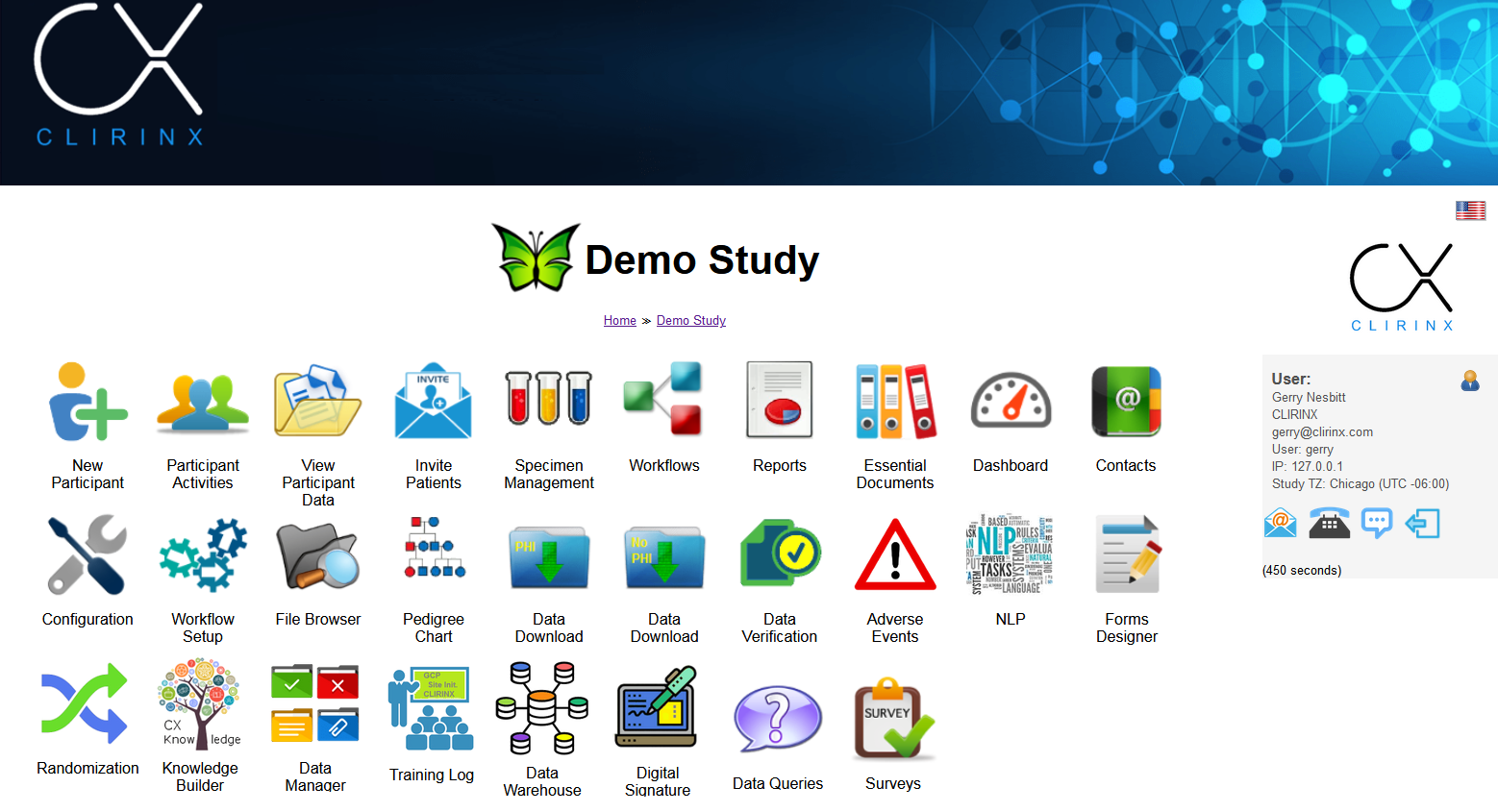
 CLIRINX EDC (Electronic Data Collection) provides a suite of software products to support web-based data collection on your clinical research study. It consists of a clinical trials management system (CTMS), a CRF/Form Designer for electronic data collection (EDC) and an electronic participant portal (ePRO).
CLIRINX EDC (Electronic Data Collection) provides a suite of software products to support web-based data collection on your clinical research study. It consists of a clinical trials management system (CTMS), a CRF/Form Designer for electronic data collection (EDC) and an electronic participant portal (ePRO).
The web-based CRF/Forms Designer is probably the most sophisticated forms designer available. It caters for the typical question types, and also has novel question types, like table-based questions (combination of text/radio/dropdown/checkbox/likert field questions in multiple fixed or dynamic rows), image mark, file upload and EEG node selection. Branching/skip rules can be implemented on individual questions, groups of questions and fields within table-based questions. And if you need a new feature or application, we can develop it for you!
Recent News
Apr 24, 2024 CLIRINX & CRID feature in new major SCN2A-related publication.
Mar 20, 2024 CRID 'Clinical Research ID' hits 4000 CRID identifiers!

