
Patient Portal
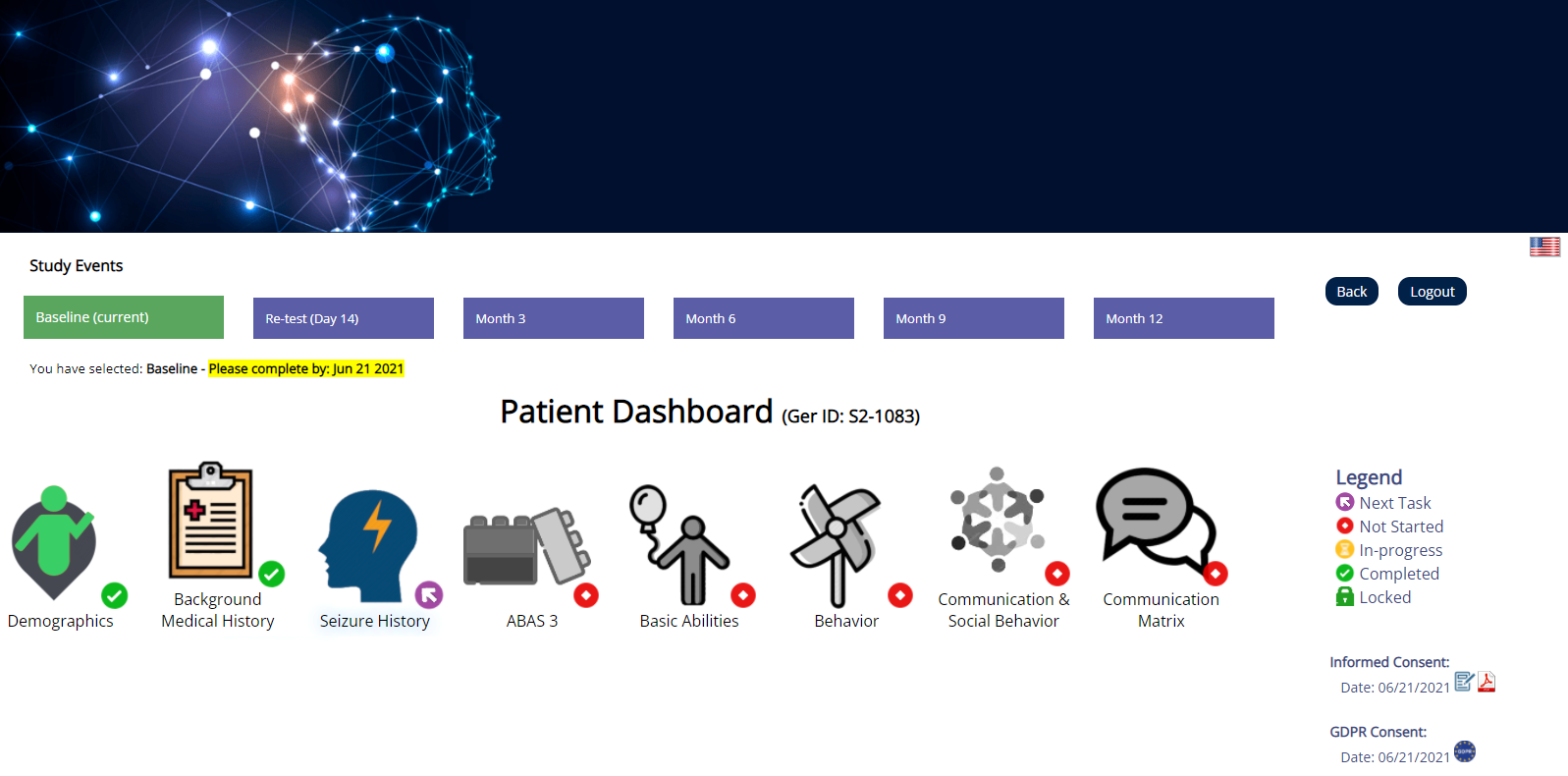
 CLIRINX Participant Portal (ePRO/EDC) is a separate website that study participants access to contribute data to the research study. It is extremely easy to use and requires no training. It allows participants to complete data collection forms, download their own data and view newsletters and summary stats on the data collected. It also facilitates diary-based data collection (daily, weekly or monthly), such as medication usage, seizures, moods, etc.
CLIRINX Participant Portal (ePRO/EDC) is a separate website that study participants access to contribute data to the research study. It is extremely easy to use and requires no training. It allows participants to complete data collection forms, download their own data and view newsletters and summary stats on the data collected. It also facilitates diary-based data collection (daily, weekly or monthly), such as medication usage, seizures, moods, etc.
The system supports multiple research studies and each research study can have its own specific URL website address. You can create a login account to access the website or login using an existing account. When you select a study for the first time, you are asked to complete the Informed Consent process (using digital signatures) for a specific study. For users in the EU/EEA, an additional consent is required to support GDPR requirements. Once the e-consent process is complete, the data collection forms are made available. The system supports data collection at multiple timepoints and multiple participants, and the user can access their own data in reports (e.g. PDF) and structured formats (e.g. CSV, JSON). The system is also integrated with CRID™ so that data can be shared, if desired, with other external research projects and PIs.
Recent News
Apr 24, 2024 CLIRINX & CRID feature in new major SCN2A-related publication.
Mar 20, 2024 CRID 'Clinical Research ID' hits 4000 CRID identifiers!

